I’ve become increasingly
concerned about the efficacy and safety of some typical nutritional supplements—here’s
why.
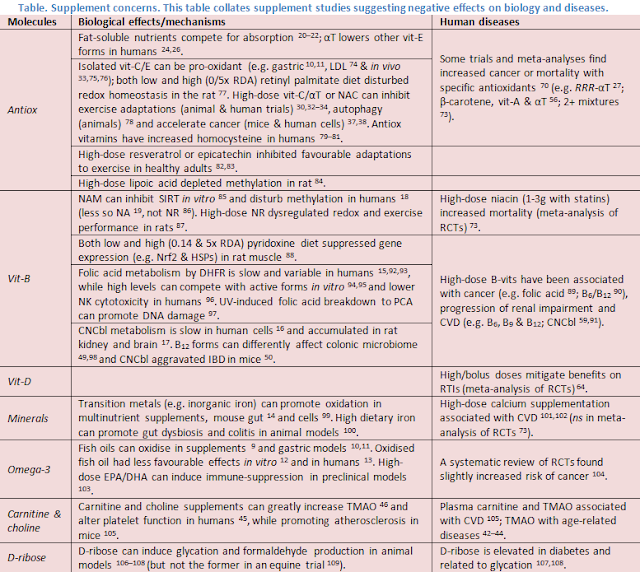
Initially, my concern is a logical appeal to nature. Food contains a complex matrix of chemicals in the balance and structure of life, to which our physiology (e.g. digestion, metabolism and microbiome) is adapted. By comparison, supplements supply concentrated food ingredients, to an extreme of isolated chemicals in unnatural forms and mega doses. My concern is fed by some studies reporting on potentially negative effects and long-term health outcomes (see table); common themes are the use of synthetic/isolated nutrients and high doses (note their use also in ME/CFS studies 1–5). Could these deviations from nature impair bioactivity or induce imbalances which limit efficacy and introduce risk? Some specific examples are discussed below.
Quality
Firstly, supplement quality regulation is variable, so products
can have varied levels of active ingredients and contaminants 6 (e.g. toxic metals 7,8). In addition, some isolated
ingredients and combinations may be prone to oxidation. For instance, fish oils
are one of the most labile supplements. Studies in different countries have
reported that many commercial fish oils are high in oxidation products,
exceeding recommended limits for standards of quality 9. Further oxidation might also occur in
the stomach, as reported in gastric models 10,11.
This may affect bioactivity. In vitro,
commercial oxidised fish oils had impaired ability to inhibit oxidation of LDL cholesterol
12, while a clinical trial
showed opposite effects on IDL/LDL composition and level 13. Similarly, multi-vit/min supplements
containing redox-active transition metals (e.g. inorganic iron and copper) and
vitamins (e.g. vit-C/E) can form free radicals and oxidise in water and gut (mouse
model), likely due to Fenton reactions 14.
Typical supplements also contain synthetic vitamins, which can be more stable,
but potentially less bioactive. For instance, folic acid (vit-B9)
and cyanocobalamin (vit-B12), despite raising blood levels, may be
less efficiently converted to active forms in tissues 15–17.
Quantity
Besides quality issues, excessive doses might also be disruptive
to cell metabolism and signalling. For instance, some nutrients are metabolised
via methylation. Consequently, high-dose niacinamide (NAM, vit-B3) can
acutely deplete betaine, elevate homocysteine and lower methylation reactions in
humans 18 (more than NA 19). Fat-soluble vitamins and
carotenoids can also compete for absorption 20–22, while unabsorbed doses may accumulate in gut cells 23. Consequently, while vit-E exists as 8
forms in nature (4 tocopherols and tocotrienols), typical alpha-tocopherol (aT)
supplementation lowers b, g and dT
in humans (i.e. RRR-aT 24,25 and all-rac-aT 26), and potentially tocotrienols 27, all of which may have unique benefits
(e.g. anti-cancer activity 28).
Further, antioxidants and oxidants function within complex redox networks, organised
in space and time to control basic cellular processes 29, suggesting potential to dysregulate
redox biology. For instance, high doses of antioxidants (e.g. vit-C + aT)
can inhibit exercise-induced redox signalling 30–32 and favourable adaptations (e.g.
insulin sensitivity, performance) in some animal and human trials 33–35. Excessive
antioxidant supplementation might also indiscriminately support pathogenic
cells (e.g. cancerous), as shown in animal models 36–38.
Supplements might also inadvertently affect the gut microbiome,
which responds rapidly to changes in diet 39
and redox 40, and influences
systemic health. For instance, microbial metabolism of TMA-containing nutrients
(e.g. choline and carnitine 41)
can fuel production of TMAO, via a meta-organismal pathway (i.e. TMA–FMO3–TMAO)
associated with age-related diseases (e.g. CVD, dementia, etc.) 42–44. Consequently, high-dose choline 45 and carnitine 46 supplements can greatly elevate TMAO (>10-fold)
and alter platelet function in humans 45.
B12 is also often taken at extremely high doses (e.g. supplements 1mg+,
diets 5–10ug 47) with limited
absorption (via active transport and passive diffusion), meaning most will pass
through the gut. B12 is normally a limited resource for gut microbes
48, while recent preclinical
studies show an ability of cyanocobalamin in particular to unfavourably modulate
the colonic microbiome 49 and exacerbate
IBD in mice 50.
Context
On the other hand, too little of any particular nutrient/metabolite
is also potentially detrimental, and may occur for various reasons. Diet is the
natural source of nutrients, though perhaps not always optimal 51. Common refined ingredients (e.g.
sugar, refined grains, oil/butter and protein isolates) provide empty calories
(macros), depleted in micro- and phytonutrients. Even whole foods can have
altered nutrient/toxin content due to industrialisation (e.g. crops 52,53 and seafood 54,55). Moreover, metabolism is affected by
genetics, diseases, ageing, etc. So appropriate supplementation might be
supportive, but when overly crude might induce its own issues; i.e. too little
or too much might be harmful—exact margins depending on individual context 56.
This may introduce further confounding into RCTs to obscure
and confuse outcomes. For instance, while the recent large VITAL trial with
prescription omega-3 did not prevent CVD (or cancer) 57, subgroup analysis showed marked benefit
in those with low fish intake 58.
A meta-analysis of CVD trials with B-vitamins linked high-dose cyanocobalamin to
harm in people with impaired renal function, obscuring benefit in those with
good function 59. Further, the
VITACOG trial of B-vitamins in MCI slowed cognitive decline and brain atrophy,
but only in those with elevated baseline homocysteine 60,61 and
omega-3 62,63, suggesting
metabolic interdependence (as
discussed previously). A meta-analysis of vit-D trials reported that daily/weekly
doses, but not bolus, prevented respiratory infections, and most prominently in
those with low baseline status 64.
In people with low antioxidant status (i.e. vit-C and GSH), supplementation has
improved exercise redox and performance 65–67,
while excessive doses may be detrimental in healthy individuals (as above). In
observational studies, higher intake and blood levels of various antioxidants are
associated with reduced mortality 68,69,
whereas in some trials and meta-analyses, typical antioxidant supplementation even
increased cancer (e.g. SELECT) or mortality (for review 70), again in relation to dose (e.g.
vit-E and β-carotene >RDA)
56 and baseline selenium levels
71.
In sum, the effects of supplements can depend upon their quality, quantity and context, all
of which may be confounding factors in trials, leading to inconsistent results
and misalignment with other lines of evidence (e.g. epidemiology and models). In
particular, nutrients are often treated like foreign drugs with linear effects,
yet they are already present in natural forms and function within metabolic networks
with complex dynamics 72. Perhaps
appreciating these nuances or adhering closer to nature could support efficacy
and safety?
Resources
References
1. Brouwers, F. M., Van Der
Werf, S., Bleijenberg, G., Van Der Zee, L. & Van Der Meer, J. W. M. The
effect of a polynutrient supplement on fatigue and physical activity of
patients with chronic fatigue syndrome: a double-blind randomized controlled
trial. QJM 95, 677–683 (2002).
2. Menon, R. et al.
Mitochondrial modifying nutrients in treating chronic fatigue syndrome: A
16-week open-label pilot study. Adv. Integr. Med. 4, 109–114
(2017).
3. Martin, R. W. Y.,
Ogston, S. A. & Evans, J. R. Effects of Vitamin and Mineral Supplementation
on Symptoms Associated with Chronic Fatigue Syndrome with Coxsackie B
Antibodies. J. Nutr. Med. 4, 11–23 (1994).
4. Montoya, J. G. et al.
KPAX002 as a treatment for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
(ME/CFS): a prospective, randomized trial. Int. J. Clin. Exp. Med. 11,
2890–2900 (2018).
5. Regland, B. et al.
Response to Vitamin B12 and Folic Acid in Myalgic Encephalomyelitis and
Fibromyalgia. PLoS One 10, e0124648 (2015).
6. Costa, J. G. et al.
Contaminants: a dark side of food supplements? Free Radic. Res. 0,
1–23 (2019).
7. Schwalfenberg, G.,
Rodushkin, I. & Genuis, S. J. Heavy metal contamination of prenatal
vitamins. Toxicol. reports 5, 390–395 (2018).
8. Genuis, S. J.,
Schwalfenberg, G., Siy, A.-K. J. & Rodushkin, I. Toxic element
contamination of natural health products and pharmaceutical preparations. PLoS
One 7, e49676 (2012).
9. Cameron-Smith, D.,
Albert, B. B. & Cutfield, W. S. Fishing for answers: is oxidation of fish
oil supplements a problem? J. Nutr. Sci. 4, e36 (2015).
10. Tirosh, O., Shpaizer, A.
& Kanner, J. Lipid Peroxidation in a Stomach Medium Is Affected by Dietary
Oils (Olive/Fish) and Antioxidants: The Mediterranean versus Western Diet. J.
Agric. Food Chem. 63, 7016–23 (2015).
11. Kanner, J. et al.
Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress
Index (POSI) for balancing nutrition and human health. Redox Biol. 12,
929–936 (2017).
12. Mason, R. P. &
Sherratt, S. C. R. Omega-3 fatty acid fish oil dietary supplements contain
saturated fats and oxidized lipids that may interfere with their intended
biological benefits. Biochem. Biophys. Res. Commun. 483, 425–429
(2017).
13. Rundblad, A., Holven, K.
B., Ottestad, I., Myhrstad, M. C. & Ulven, S. M. High-quality fish oil has
a more favourable effect than oxidised fish oil on intermediate-density
lipoprotein and LDL subclasses: a randomised controlled trial. Br. J. Nutr.
117, 1291–1298 (2017).
14. Rabovsky, A. B., Buettner,
G. R. & Fink, B. In vivo imaging of free radicals produced by
multivitamin-mineral supplements. BMC Nutr. 1, 1–9 (2015).
15. Scaglione, F. &
Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same
thing. Xenobiotica. 44, 480–8 (2014).
16. Hall, C., Begley, J. &
Green-Colligan, P. The availability of therapeutic hydroxocobalamin to cells. Blood
63, 335–341 (1984).
17. Greibe, E. et al.
The tissue profile of metabolically active coenzyme forms of vitamin B12
differs in vitamin B12-depleted rats treated with hydroxo-B12 or cyano-B12. Br.
J. Nutr. 120, 49–56 (2018).
18. Sun, W.-P. et al.
Excess nicotinamide inhibits methylation-mediated degradation of catecholamines
in normotensives and hypertensives. Hypertens. Res. 35, 180–5
(2012).
19. Sun, W.-P. et al.
Comparison of the effects of nicotinic acid and nicotinamide degradation on
plasma betaine and choline levels. Clin. Nutr. 36, 1136–1142
(2017).
20. Reboul, E. et al.
Effect of the main dietary antioxidants (carotenoids, gamma-tocopherol,
polyphenols, and vitamin C) on alpha-tocopherol absorption. Eur. J. Clin.
Nutr. 61, 1167–73 (2007).
21. Reboul, E. et al.
Differential effect of dietary antioxidant classes (carotenoids, polyphenols,
vitamins C and E) on lutein absorption. Br. J. Nutr. 97, 440–6
(2007).
22. Reboul, E. Vitamin e bioavailability:
Mechanisms of intestinal absorption in the spotlight. Antioxidants 6,
(2017).
23. Reboul, E. The gut: a
regulatory hall governing fat-soluble micronutrient absorption. Am. J. Clin.
Nutr. (2019). doi:10.1093/ajcn/nqz199
24. Gutierrez, A. D., de
Serna, D. G., Robinson, I. & Schade, D. S. The response of gamma vitamin E
to varying dosages of alpha vitamin E plus vitamin C. Metabolism. 58,
469–78 (2009).
25. Huang, H.-Y. & Appel,
L. J. Supplementation of diets with alpha-tocopherol reduces serum concentrations
of gamma- and delta-tocopherol in humans. J. Nutr. 133, 3137–40
(2003).
26. Mondul, A. M. et al.
Serum Metabolomic Response to Long-Term Supplementation with
all-rac-α-Tocopheryl Acetate in a Randomized Controlled Trial. J. Nutr.
Metab. 2016, 6158436 (2016).
27. Gee, P. T. Unleashing the
untold and misunderstood observations on vitamin E. Genes Nutr. 6,
5–16 (2011).
28. Jiang, Q. Natural Forms of
Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr.
8, 850–867 (2017).
29. Jones, D. P. & Sies,
H. The Redox Code. Antioxid. Redox Signal. 23, 734–46 (2015).
30. Done, A. J. & Traustadóttir,
T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 10,
191–199 (2016).
31. Ristow, M. &
Schmeisser, K. Mitohormesis: Promoting Health and Lifespan by Increased Levels
of Reactive Oxygen Species (ROS). Dose. Response. 12, 288–341
(2014).
32. Merry, T. L. & Ristow,
M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates
exercise-induced mitochondrial biogenesis and the anti-oxidant response in
mice. J. Physiol. 594, 5195–207 (2016).
33. Nikolaidis, M. G.,
Kerksick, C. M., Lamprecht, M. & McAnulty, S. R. Does vitamin C and E
supplementation impair the favorable adaptations of regular exercise? Oxid.
Med. Cell. Longev. 2012, 707941 (2012).
34. Merry, T. L. & Ristow,
M. Do antioxidant supplements interfere with skeletal muscle adaptation to
exercise training? J. Physiol. 594, 5135–47 (2016).
35. Gomez-Cabrera, M. C.,
Salvador-Pascual, A., Cabo, H., Ferrando, B. & Vina, J. Redox modulation of
mitochondriogenesis in exercise. Does antioxidant supplementation blunt the
benefits of exercise training? Free Radic. Biol. Med. 86, 37–46
(2015).
36. Cockfield, J. A. &
Schafer, Z. T. Antioxidant Defenses: A Context-Specific Vulnerability of Cancer
Cells. Cancers (Basel). 11, 1208 (2019).
37. Sayin, V. I. et al.
Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med.
6, 221ra15 (2014).
38. Le Gal, K. et al.
Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med.
7, 308re8 (2015).
39. David, L. a et al.
Diet rapidly and reproducibly alters the human gut microbiome. Nature 505,
559–63 (2014).
40. Rivera-Chávez, F., Lopez,
C. A. & Bäumler, A. J. Oxygen as a driver of gut dysbiosis. Free Radic.
Biol. Med. 105, 93–101 (2017).
41. Li, X. S. et al.
Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient
precursor, as a predictor of incident cardiovascular disease risk. JCI
insight 3, 1–18 (2018).
42. Vogt, N. M. et al.
The gut microbiota-derived metabolite trimethylamine N -oxide is elevated in
Alzheimer’s disease. Alzheimer’s Res. Ther. 2018 101 10, 124
(2018).
43. Brunt, V. E. et al.
Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and
Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 76,
101–112 (2020).
44. Randrianarisoa, E. et
al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early
Atherosclerosis in Humans. Sci. Rep. 6, 26745 (2016).
45. Zhu, W., Wang, Z., Tang,
W. H. W. & Hazen, S. L. Gut Microbe-Generated Trimethylamine N-Oxide From
Dietary Choline Is Prothrombotic in Subjects. Circulation 135,
1671–1673 (2017).
46. Vallance, H. D. et al.
Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with
mitochondrial disorders treated with oral l-carnitine. Mol. Genet. Metab.
reports 15, 130–133 (2018).
47. Kraft, T. S. et al.
Nutrition transition in 2 lowland Bolivian subsistence populations. Am. J.
Clin. Nutr. 108, 1183–1195 (2018).
48. Rowley, C. A. &
Kendall, M. M. To B12 or not to B12: Five questions on the role of cobalamin in
host-microbial interactions. PLoS Pathog. 15, e1007479 (2019).
49. Xu, Y. et al.
Cobalamin (Vitamin B12) Induced a Shift in Microbial Composition and Metabolic
Activity in an in vitro Colon Simulation. Front. Microbiol. 9,
2780 (2018).
50. Zhu, X. et al.
Impact of Cyanocobalamin and Methylcobalamin on Inflammatory Bowel Disease and
the Intestinal Microbiota Composition. J. Agric. Food Chem. 67,
916–926 (2019).
51. Kennedy, D. O. et al.
Multivitamins and minerals modulate whole-body energy metabolism and cerebral
blood-flow during cognitive task performance: a double-blind, randomised,
placebo-controlled trial. Nutr. Metab. (Lond). 13, 11 (2016).
52. Barański, M. et al.
Higher antioxidant and lower cadmium concentrations and lower incidence of
pesticide residues in organically grown crops: a systematic literature review
and meta-analyses. Br. J. Nutr. 112, 1–18 (2014).
53. Crinnion, W. J. Organic Foods
Contain Higher Levels of Certain Nutrients, Lower Levels of Pesticides, and May
Provide Health Benefits for the Consumer. Altern. Med. Rev. 15,
(2010).
54. Shi, L. et al.
Joint Analysis of Metabolite Markers of Fish Intake and Persistent Organic
Pollutants in Relation to Type 2 Diabetes Risk in Swedish Adults. J. Nutr.
149, 1413–1423 (2019).
55. Donat-Vargas, C. et al.
Cardiovascular and cancer mortality in relation to dietary polychlorinated
biphenyls and marine polyunsaturated fatty acids: a nutritional-toxicological
aspect of fish consumption. J. Intern. Med. 287, 197–209 (2020).
56. Bjelakovic, G., Nikolova,
D. & Gluud, C. Meta-regression analyses, meta-analyses, and trial
sequential analyses of the effects of supplementation with beta-carotene, vitamin
A, and vitamin E singly or in different combinations on all-cause mortality: do
we have evidence for lack of harm? PLoS One 8, e74558 (2013).
57. Manson, J. E. et al.
Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N.
Engl. J. Med. 380, 23–32 (2019).
58. Kris-Etherton, P. M. et
al. Recent Clinical Trials Shed New Light on the Cardiovascular Benefits of
Omega-3 Fatty Acids. Methodist Debakey Cardiovasc. J. 15, 171–178
(2019).
59. Spence, J. D., Yi, Q.
& Hankey, G. J. B vitamins in stroke prevention: time to reconsider. Lancet
Neurol. 16, 750–760 (2017).
60. Douaud, G. et al.
Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin
treatment. Proc. Natl. Acad. Sci. 110, 9523–8 (2013).
61. de Jager, C. A., Oulhaj,
A., Jacoby, R., Refsum, H. & Smith, A. D. Cognitive and clinical outcomes
of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a
randomized controlled trial. Int. J. Geriatr. Psychiatry 27,
592–600 (2012).
62. Oulhaj, A., Jernerén, F.,
Refsum, H., Smith, A. D. & de Jager, C. A. Omega-3 Fatty Acid Status
Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive
Impairment. J. Alzheimers. Dis. 50, 547–57 (2016).
63. Jerneren, F. et al.
Brain atrophy in cognitively impaired elderly: the importance of long-chain -3
fatty acids and B vitamin status in a randomized controlled trial. Am. J.
Clin. Nutr. 102, 215–21 (2015).
64. Martineau, A. R. et al.
Vitamin D supplementation to prevent acute respiratory tract infections: Systematic
review and meta-analysis of individual participant data. BMJ 356,
(2017).
65. Paschalis, V., Theodorou,
A. A., Margaritelis, N. V, Kyparos, A. & Nikolaidis, M. G. N-acetylcysteine
supplementation increases exercise performance and reduces oxidative stress
only in individuals with low levels of glutathione. Free Radic. Biol. Med.
115, 288–297 (2018).
66. Johnston, C. S., Corte, C.
& Swan, P. D. Marginal vitamin C status is associated with reduced fat
oxidation during submaximal exercise in young adults. Nutr. Metab. (Lond).
3, 35 (2006).
67. Paschalis, V. et al.
Low vitamin C values are linked with decreased physical performance and
increased oxidative stress: reversal by vitamin C supplementation. Eur. J.
Nutr. 55, 45–53 (2016).
68. Jayedi, A., Rashidy-Pour,
A., Parohan, M., Zargar, M. S. & Shab-Bidar, S. Dietary Antioxidants,
Circulating Antioxidant Concentrations, Total Antioxidant Capacity, and Risk of
All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of
Prospective Observational Studies. Adv. Nutr. 9, 701–716 (2018).
69. Parohan, M. et al.
Dietary total antioxidant capacity and mortality from all causes,
cardiovascular disease and cancer: a systematic review and dose-response
meta-analysis of prospective cohort studies. Eur. J. Nutr. 58,
2175–2189 (2019).
70. Bjelakovic, G., Nikolova,
D. & Gluud, C. Antioxidant supplements and mortality. Curr. Opin. Clin.
Nutr. Metab. Care 17, 40–4 (2014).
71. Kristal, A. R. et al.
Baseline selenium status and effects of selenium and vitamin e supplementation
on prostate cancer risk. J. Natl. Cancer Inst. 106, djt456
(2014).
72. Grant, W. B., Boucher, B.
J., Bhattoa, H. P. & Lahore, H. Why vitamin D clinical trials should be
based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol.
177, 266–269 (2018).
73. Jenkins, D. J. A. et
al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. Journal
of the American College of Cardiology 71, 2570–2584 (2018).
74. Ohkawa, S. et al.
Pro-oxidative effect of alpha-tocopherol in the oxidation of LDL isolated from
co-antioxidant-depleted non-diabetic hemodialysis patients. Atherosclerosis
176, 411–8 (2004).
75. Pearson, P., Lewis, S. A.,
Britton, J., Young, I. S. & Fogarty, A. The pro-oxidant activity of
high-dose vitamin E supplements in vivo. BioDrugs 20, 271–3
(2006).
76. Poulsen, H. E. et al.
Does vitamin C have a pro-oxidant effect? Nature 395, 231–232
(1998).
77. Cha, J.-H., Yu, Q.-M.
& Seo, J.-S. Vitamin A supplementation modifies the antioxidant system in
rats. Nutr. Res. Pract. 10, 26–32 (2016).
78. Underwood, B. R. et al.
Antioxidants can inhibit basal autophagy and enhance neurodegeneration in
models of polyglutamine disease. Hum. Mol. Genet. 19, 3413–29
(2010).
79. Woodside, J. V et al.
Effect of B-group vitamins and antioxidant vitamins on hyperhomocysteinemia: a
double-blind, randomized, factorial-design, controlled trial. Am. J. Clin.
Nutr. 67, 858–66 (1998).
80. Cafolla, A. et al.
Effect of folic acid and vitamin C supplementation on folate status and
homocysteine level: a randomised controlled trial in Italian smoker-blood
donors. Atherosclerosis 163, 105–11 (2002).
81. Ullegaddi, R., Powers, H.
J. & Gariballa, S. E. Antioxidant supplementation with or without B-group
vitamins after acute ischemic stroke: a randomized controlled trial. JPEN.
J. Parenter. Enteral Nutr. 30, 108–14 (2006).
82. Schwarz, N. A. et al.
(-)-Epicatechin Supplementation Inhibits Aerobic Adaptations to Cycling
Exercise in Humans. Front. Nutr. 5, 132 (2018).
83. Gliemann, L., Nyberg, M.
& Hellsten, Y. Effects of exercise training and resveratrol on vascular
health in aging. Free Radic. Biol. Med. 98, 165–176 (2016).
84. Stabler, S. P., Sekhar,
J., Allen, R. H., O’Neill, H. C. & White, C. W. Alpha-lipoic acid induces
elevated S-adenosylhomocysteine and depletes S-adenosylmethionine. Free
Radic. Biol. Med. 47, 1147–53 (2009).
85. Hwang, E. S. & Song,
S. B. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator
in cells. Cell. Mol. Life Sci. 74, 3347–3362 (2017).
86. Conze, D., Brenner, C.
& Kruger, C. L. Safety and Metabolism of Long-term Administration of NIAGEN
(Nicotinamide Riboside Chloride) in a Randomized, Double-Blind,
Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep.
9, 9772 (2019).
87. Kourtzidis, I. A. et
al. Nicotinamide riboside supplementation dysregulates redox and energy
metabolism in rats: Implications for exercise performance. Exp. Physiol.
103, 1357–1366 (2018).
88. Suidasari, S., Uragami,
S., Yanaka, N. & Kato, N. Dietary vitamin B6 modulates the gene expression
of myokines, Nrf2-related factors, myogenin and HSP60 in the skeletal muscle of
rats. Exp. Ther. Med. 14, 3239–3246 (2017).
89. Kim, Y. I. Folate and
cancer: A tale of Dr. Jekyll and Mr. Hyde? Am. J. Clin. Nutr. 107,
139–142 (2018).
90. Brasky, T. M., White, E.
& Chen, C.-L. Long-Term, Supplemental, One-Carbon Metabolism-Related
Vitamin B Use in Relation to Lung Cancer Risk in the Vitamins and Lifestyle
(VITAL) Cohort. J. Clin. Oncol. 35, 3440–3448 (2017).
91. Spence, J. D. Harm With High
Levels of Serum B12 in Elderly Persons. J. Gerontol. A. Biol. Sci. Med. Sci.
74, 137 (2019).
92. Bailey, S. W. &
Ayling, J. E. The extremely slow and variable activity of dihydrofolate
reductase in human liver and its implications for high folic acid intake. Proc.
Natl. Acad. Sci. U. S. A. 106, 15424–9 (2009).
93. Patanwala, I. et al.
Folic acid handling by the human gut: implications for food fortification and
supplementation. Am. J. Clin. Nutr. 100, 593–9 (2014).
94. Smith, D. E. C., Hornstra,
J. M., Kok, R. M., Blom, H. J. & Smulders, Y. M. Folic acid supplementation
does not reduce intracellular homocysteine, and may disturb intracellular
one-carbon metabolism. Clin. Chem. Lab. Med. 51, 1643–50 (2013).
95. Smith, D. et al.
Folic Acid Impairs the Uptake of 5-Methyltetrahydrofolate in Human Umbilical
Vascular Endothelial Cells. J. Cardiovasc. Pharmacol. 70, 271–275
(2017).
96. Paniz, C. et al. A
Daily Dose of 5 mg Folic Acid for 90 Days Is Associated with Increased Serum
Unmetabolized Folic Acid and Reduced Natural Killer Cell Cytotoxicity in
Healthy Brazilian Adults. J. Nutr. 147, 1677–1685 (2017).
97. Offer, T. et al.
5-Methyltetrahydrofolate inhibits photosensitization reactions and strand
breaks in DNA. FASEB J. 21, 2101–7 (2007).
98. Kelly, C. J. et al.
Oral vitamin B12 supplement is delivered to the distal gut, altering the
corrinoid profile and selectively depleting Bacteroides in C57BL/6 mice. Gut
Microbes 10, 654–662 (2019).
99. Bergström, T., Ersson, C.,
Bergman, J. & Möller, L. Vitamins at physiological levels cause oxidation
to the DNA nucleoside deoxyguanosine and to DNA--alone or in synergism with
metals. Mutagenesis 27, 511–7 (2012).
100. Mahalhal, A. et al.
Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS
One 13, e0202460 (2018).
101. Li, S. et al.
Long-term calcium supplementation may have adverse effects on serum cholesterol
and carotid intima-media thickness in postmenopausal women: a double-blind,
randomized, placebo-controlled trial. Am. J. Clin. Nutr. 98, 1353–9
(2013).
102. Tankeu, A. T., Ndip Agbor,
V. & Noubiap, J. J. Calcium supplementation and cardiovascular risk: A
rising concern. J. Clin. Hypertens. (Greenwich). 19, 640–646
(2017).
103. Fenton, J. I., Hord, N. G.,
Ghosh, S. & Gurzell, E. a. Immunomodulation by dietary long chain omega-3
fatty acids and the potential for adverse health outcomes. Prostaglandins.
Leukot. Essent. Fatty Acids 89, 379–90 (2013).
104. Hanson, S., Thorpe, G.,
Winstanley, L., Abdelhamid, A. S. & Hooper, L. Omega-3, omega-6 and total
dietary polyunsaturated fat on cancer incidence: systematic review and
meta-analysis of randomised trials. Br. J. Cancer 122, 1260–1270
(2020).
105. Koeth, R. A. et al.
Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat,
promotes atherosclerosis. Nat. Med. 19, 576–85 (2013).
106. Wang, Y. et al.
Formaldehyde produced from d-ribose under neutral and alkaline conditions. Toxicol.
reports 6, 298–304 (2019).
107. Chen, Y. et al.
d-Ribose contributes to the glycation of serum protein. Biochim. Biophys.
acta. Mol. basis Dis. 1865, 2285–2292 (2019).
108. Yu, L. et al.
D-ribose is elevated in T1DM patients and can be involved in the onset of
encephalopathy. Aging (Albany. NY). 11, 4943–4969 (2019).
109. Sinatra, S. T. &
Caiazzo, C. (D)-Ribose supplementation in the equine: lack of effect on
glycated plasma proteins suggesting safety in humans. J. Am. Coll. Nutr.
34, 108–12 (2015).
No comments:
Post a Comment