Low carbohydrate (carb) diets are advocated for all kinds of
health conditions (incl. ME/CFS), by Atkins/weight-loss/Paleo movements and some alternative MDs. These movements demonise carbs and oversimplify their role in health and disease. So here is a reappraisal of the humble
carb.
 Carbohydrates
(carbon hydrates) are molecules composed of carbon, hydrogen and oxygen, which
are present in nature as various different simple sugars (monosaccharides) and
chains of sugars (oligo- and polysaccharides). Carbohydrates are principally synthesised
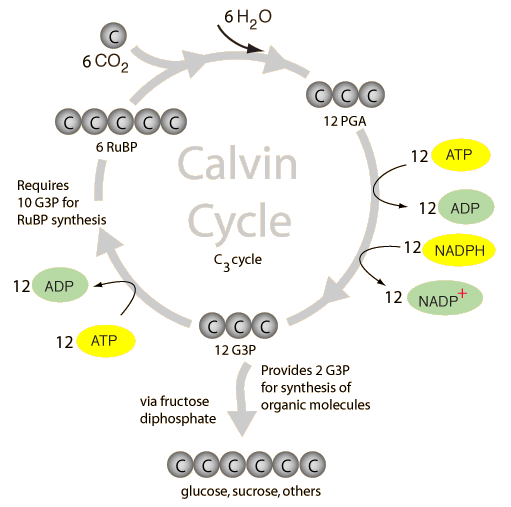
by photosynthetic organisms, which on land are plants. Photosynthesis essentially converts
light energy into chemical energy, which can then be stored in sugars/carbohydrates.
Initially light photons are used to power an electron transport chain which uses
H2O and generates NADPH and ATP, these are then used in the Calvin
cycle (with CO2-derived carbon fixation) to synthesise glucose (C6H12O6)
and other sugars. Simple sugars are combined into polysaccharides for energy
storage (starch) and cell structure (e.g. cellulose), as well as contributing
to protein and fat synthesis. If we zoom out, then on a macro scale plants play a fundamental role in the carbon cycle on earth by fixing atmospheric carbon (from
CO2) into carbohydrates and related molecules, which then pass
through the entire animal kingdom – plants are eaten by herbivores, which are
eaten by carnivores.
Carbohydrates
(carbon hydrates) are molecules composed of carbon, hydrogen and oxygen, which
are present in nature as various different simple sugars (monosaccharides) and
chains of sugars (oligo- and polysaccharides). Carbohydrates are principally synthesised
by photosynthetic organisms, which on land are plants. Photosynthesis essentially converts
light energy into chemical energy, which can then be stored in sugars/carbohydrates.
Initially light photons are used to power an electron transport chain which uses
H2O and generates NADPH and ATP, these are then used in the Calvin
cycle (with CO2-derived carbon fixation) to synthesise glucose (C6H12O6)
and other sugars. Simple sugars are combined into polysaccharides for energy
storage (starch) and cell structure (e.g. cellulose), as well as contributing
to protein and fat synthesis. If we zoom out, then on a macro scale plants play a fundamental role in the carbon cycle on earth by fixing atmospheric carbon (from
CO2) into carbohydrates and related molecules, which then pass
through the entire animal kingdom – plants are eaten by herbivores, which are
eaten by carnivores.
Glucose fuels energy, redox
and biosynthesis
In
animals all dietary carbohydrates must first be broken down into their
respective simple sugar components before being absorbed. By far the most
important sugar to human nutrition is glucose. Glucose enters cells via GLUT
transporters and is rapidly converted to glucose-6-phosphate before fuelling several
metabolic pathways.
Oxidation
of glucose via glycolysis yields high
energy molecules (NADH and ATP) and pyruvate, which can be fully oxidised in
mitochondria for more ATP (note cellular respiration releases CO2
back into the environment). By weight glucose oxidation generates less ATP than
lipid oxidation (fats contain more hydrogen), however despite this there is an
organ, cell and activity-specific preference in the body. Glucose is a primary fuel for brain, liver, active muscle, activated immune cells, red blood cells
and developing foetus 1–3. Whereas other tissues mainly use fats such
as heart, kidney, resting muscle and quiescent immune cells. Why this difference? Perhaps
because glucose uptake and cytosolic metabolism is quick; by contrast lipid β-oxidation
occurs in mitochondria (and peroxisomes for long chain fats), demands more
oxygen and generates more reactive oxygen species
(ROS) 1,4. Cells benefiting from these types of differences
may favour glucose as fuel.
In
parallel to glycolysis, glucose feeds the pentose phosphate pathway (PPP). The
first part of this pathway oxidises glucose to provide electrons for NADPH,
which maintains cellular redox/glutathione homeostasis and reductive/lipid
biosynthesis. The second and non-oxidative part of the PPP generates pentose/ribose
and erythrose sugars for nucleotide (and therefore ATP, FAD, NAD, RNA, DNA) and
aromatic amino acid synthesis respectively. Glucose flux through glycolysis vs.
PPP is regulated by cellular redox. Oxidative stress inhibits glycolysis,
while triggering an Nrf2-dependent expression of PPP enzymes to restore redox
homeostasis 5,6. Notably in most animals glucose is also the
substrate for ascorbic acid (vitamin C, C6H8O6)
synthesis, although humans can no longer catalyse the last step due to a non-functional
gulonolactone oxidase gene. This may not be such a bad thing since production
of ascorbic acid consumes glucose and generates ROS.
Less
often appreciated is that sugars and glycosylation (sugar
addition) are required for the synthesis of many basic glycan-containing molecules, such as glycoproteins
(e.g. mucins, immunoglobulins, etc.), glycophospholipids and glycosaminoglycans
(e.g. chondroitin, hyaluronan, etc.). In fact glycans are the most abundant
molecules in the body; 1-2% of the human genome encodes proteins involved in
glycan formation! Of central importance is glucose metabolism through the hexosamine biosynthetic
pathway, which generates sugar-amines (N-acetylgalactosamine
and N-acetylglucosamine) used to build glycoconjugates 7. Finally glycosylation is analogous to
phosphorylation in that it is a dynamic post-translational modification which regulates
the activity of many cellular, nuclear and mitochondrial proteins.
Glucose homeostasis
Blood
glucose is maintained within a tight range (e.g. 4.4-6.1 mmol/L) to ensure consistent
supply to tissues. Postprandial (after meal) elevations in blood glucose stimulates
insulin release which increases uptake into muscle and adipose tissue (via
GLUT4), and also inhibits liver glucose production. Surplus glucose is combined
in glycogenesis to form the polysaccharide glycogen (animal starch) which
serves as a short-term glucose store (mainly in liver and muscle). Dietary fructose
also contributes to liver stores via metabolism by fructolysis to glucose and glycogen. Glucose can further be converted
to fat by lipogenesis for longer term storage or metabolism.
Various
stimuli can trigger glucose release back into blood. During periods of stress the
glucocorticoid cortisol promotes glycogenolysis and gluconeogenesis to increase
blood glucose levels. Similarly under conditions of dietary carbohydrate
deprivation, glycogen depletion and low blood glucose/insulin levels,
compensatory pathways are activated such as gluconeogenesis and ketogenesis. Hepatic
gluconeogenesis is required to supply new glucose to maintain blood levels,
while ketogenesis converts acetyl-CoA (from lipid oxidation) to ketone bodies
which provide an additional fuel for several tissues (esp. brain), thereby
sparing blood glucose (which must not drop too low). Note ketones also act as
signaling molecules to achieve metabolic adaptation, which involves suppression
of sympathetic activity to lower resting energy usage 8,9.
Gut microbiome
Use
of sugars as fuel is conserved throughout life and as such carbohydrates feed
the gut microbiota. Gut microbes typically have a glycolytic metabolism, with
some being capable of various forms of aerobic or anaerobic respiration; however
the near anoxic conditions of the gut mean a fermentative anaerobic metabolism
dominates. Fermentation does not completely oxidise sugars and therefore generates
waste products such as ethanol, lactate and short chain fatty acids (SCFAs),
which can be salvaged by the body for energy via cellular respiration.
Carbohydrates
available to the gut microbiota come from both host and diet. The gut lining (and
other internal surfaces) are made up of epithelial cell barriers coated in
mucus secreted by goblet cells. Mucus is made from mucin, a heavily
glycosylated (sugar-coated) protein composed of 80% carbohydrate 10 (e.g. N-acetylgalactosamine,
N-acetylglucosamine, fucose, galactose, sialic acid, etc.). Mucin carbohydrate
synthesis occurs in the goblet cell golgi complex and requires glucose 11 (hexosamine pathway above). Mucus acts to shield
the epithelium 10 and maintain immune tolerance to antigen 12. In addition the outer mucus layer provides
adherence and food for many beneficial microbes (e.g. Akkermansia, butyrate-producing bacteria, Lactobacilli and Bifidobacteria)
13–15. Furthermore recently it was reported that
systemic immune activation induces rapid fucosylation of small intestinal cells,
which presents fucose directly to the gut microbiota and maintains
microbiota-host homeostasis 16.
Diet
provides carbohydrates to the gut microbiota in the form of simple sugars and
complex polysaccharides; note sugar-related molecules such as ascorbate
(vitamin C) can also be readily fermented 17,18. Indigestible carbohydrate polysaccharides (resistant
starch and fibre) pass into the colon where bacterial fermentation generates lactate
and SCFAs. These molecules have a myriad of beneficial effects including: acidify
colon, inhibit pathogens (e.g. Enterobacteriaceae and Candida), enhance mineral
absorption, feed colonocytes, provide energy to the host, and favourably
regulate systemic metabolism, immunity and neurobiology. In particular carbohydrate
fermentation regulates systemic energy metabolism via multiple mechanisms
including: increased satiety, fat metabolism (adipose tissues) and improved
insulin sensitivity and glucose tolerance, all of which counter the metabolic
syndrome phenotype 9,19,20. Ultimately we seem well adapted to a carbohydrate-based
(saccharolytic) colonic fermentation; note this is not the case for high fat or
protein diets 21–23!
Diet and evolution
Carbohydrates
have probably played a fundamental role in human evolution 2. Humans have evolved from a mainly herbivorous
anthropoid lineage, with our closest living relative, chimps (also Hominidae
family), being omnivorous frugivores
(prefer fruit). One of several key genes which separates us from
them encodes amylase 24,25, an enzyme enabling us to digest starches,
suggesting a broadening of our diet to include starchy plant foods. Another key
evolutionary change is our relatively large brain and small gut and teeth. This
is thought to be the result of an increase in food quality, due to greater
consumption of meat, food processing and cooking 26, which makes both animal and plant foods far
easier to digest and extract energy from 27,28.
In
particular the brains preference for using glucose, suggests at some point
humans must have started consuming more carbohydrates to meet this increased
metabolic demand (at rest the brain consumes 20% of blood oxygen and 25% of
blood glucose). The major dietary source of glucose is likely to have been the
starch in plant tubers 2, which is rendered highly digestible after
cooking 27,28.Therefore a convergence in cooking practices,
starch consumption and amylase may have been pivotal in allowing the
enlargement of our brains 2. Also crucial would have been increased
consumption of marine life rich in the omega-3 fatty acid DHA 29, which is the major structural fatty acid in
the brain, cannot be efficiently synthesised endogenously, and is positively
coupled to glucose transport and metabolism 30,31.
Research
on modern hunter-gatherers also indicates that carbohydrates can make up a
major portion of their diets, especially in populations from warm climates
(e.g. !Kung, Yanomamo and Hadza) 32,33. For instance the Hadza people of Tanzania,
Africa (where humans initially evolved) get around 68% of their kcal from
plants, including baobab (14%), honey (15%), tubers (19%) and berries (20%) 34. Note honey is a favourite food of many
hunter-gatherers 34 – they are human after all!
However, on the flip side, there is the older view that Palaeolithic humans ate mostly meat. This stems from old ethnographic data 32 and archaeological studies which are unfortunately limited by methodological issues and biases favouring markers of meat consumption (see). Even the idea that Neanderthals only ever ate meat has now been overturned by modern research on dental calculus and old poop (refs in 35). However people living in extreme cold environments such as the Arctic, where there are few plants, certainly eat an almost entirely animal-based diet. Although even here they may consume 15-20% of calories as carbohydrate (they are not in ketosis), mainly in the form of glycogen which is persevered in animal muscle at low temperatures 2. Moreover Inuit peoples have enlarged livers 2 and recently genetic adaptations have been identified in circum-Arctic populations which enable a greater rate of lipid oxidation (as well as having other deleterious effects) 36.
However, on the flip side, there is the older view that Palaeolithic humans ate mostly meat. This stems from old ethnographic data 32 and archaeological studies which are unfortunately limited by methodological issues and biases favouring markers of meat consumption (see). Even the idea that Neanderthals only ever ate meat has now been overturned by modern research on dental calculus and old poop (refs in 35). However people living in extreme cold environments such as the Arctic, where there are few plants, certainly eat an almost entirely animal-based diet. Although even here they may consume 15-20% of calories as carbohydrate (they are not in ketosis), mainly in the form of glycogen which is persevered in animal muscle at low temperatures 2. Moreover Inuit peoples have enlarged livers 2 and recently genetic adaptations have been identified in circum-Arctic populations which enable a greater rate of lipid oxidation (as well as having other deleterious effects) 36.
For
more on all this check out these interesting talks by hunter-gatherer/paleo
researchers: Christina Warinner, Alyssa Crittenden, Richard Wrangham and Nathaniel Dominy. And also the ‘Evolution of diet’ page at
National Geographic.
Modern diets
A
high consumption of plants, carbohydrates and fibre is a key feature of healthy
populations including Mediterranean 37, Okinawan 38 and Kitavan diets 39. In fact people living in areas of the world
with the best health and longevity statistics (i.e. blue zones) consume semi-vegetarian diets. By contrast
western industrialised diets are widely considered to contribute to chronic
disease (e.g. dental/periodontal, metabolic, inflammatory, degenerative and
skin diseases, amongst others). The so called ‘western diet’ is high in refined
carbohydrates/sugar, fat (saturated and omega-6), meat, empty calories and
additives, while being low in whole plant foods/fibre, omega-3 and
micronutrients.
Similar
to other nutrients, carbohydrates start to become unhealthy when they are
processed and refined. Food processing removes beneficial dietary fibre and
adds sugar/fructose syrup. This results in high glycaemic index (GI) foods which
promote exaggerated elevations in blood glucose and challenge energy
homeostasis, while the lack of fibre starves the gut microbiota and prevents
its ability to promote heath. Western diets may also be relatively high in
harmful advanced glycation end-products (AGEs, aka. glycotoxins). AGEs form
when sugars react with free amino acids, fats and nucleic acids. In the body,
AGE formation may be promoted by elevated glucose (and more so fructose) and
oxidative stress. In the diet, AGEs are formed in heat-treated foods,
especially those of animal origin, which greatly contribute to body pools. AGEs
activate the receptor for AGE (RAGE) and generally act to promote oxidative
stress, inflammation and chronic disease.
So when
considering our biology, microbiome, evolution and current health epidemiology,
it may be changes in carbohydrate quality
rather than quantity which are most
important. Note a similar quality/balance paradigm is also accepted for fats:
saturated/unsaturated, omega-3/6, etc.
Modern diseases
Many
modern diseases involve problems with carbohydrate metabolism. For instance systemic
insulin resistance and glucose intolerance is a characteristic of metabolic
syndrome and diabetes. Brain insulin resistance and glucose hypometabolism is a
hallmark of Alzheimer’s disease (aka. diabetes type-3). Impaired glucose
metabolism has also recently been reported in ME/CFS blood and muscle tissue 40,41. In these conditions, low GI or even low-carb
diets may be required to prevent the negative effects of poor glucose control,
while other macronutrients (protein and fats/ketones) can supply alternative
fuel when necessary 30. Low carb diets are also effective for weight
loss (as is any calorie-restricted diet) and can improve cardiometabolic markers. However these approaches are
compensatory and not necessarily going to achieve the best health. In
humans, low carb diets 42 and higher animal protein
intake 43 have been associated with
increased disease and mortality. Furthermore, if mice are anything to go by, then
they
have the best cardiometabolic health, aging and longevity when fed an ad libitum low protein, high carb diet 44. This seems reminiscent of the diet of blue
zone populations.
So
what really causes problems with carbohydrate metabolism? Perhaps of foremost
importance, most aspects of the western diet can promote insulin resistance,
including increased levels of sugar (esp. industrial fructose), fat (esp. sat
fat), AGEs, artificial sweeteners and emulsifiers, and low levels of fibre,
polyphenols, omega-3 and micronutrients (esp. magnesium 45).
Interestingly,
most things which screw up glucose tolerance, do so by inducing gut dysbiosis
and inflammation 46–49. Alterations to the gut microbiota, such as small
intestinal bacterial overgrowth (SIBO) or gut dysbiosis, occur in most chronic
disorders. Again low-carb diets are often advocated here, since this might broadly
lower microbe growth, and therefore SIBO 50 and theoretically the presence of some pathogens
51. Certainly lowering specific fermentable
carbohydrates (FODMAPs) improves symptoms in irritable bowel syndrome (IBS),
which is frequently associated with SIBO. However low-carb diets seem unlikely to
treat the causes of SIBO and dysbiosis, such as antibiotic use, western diet, digestive
insufficiency, immunodeficiency and slow intestinal motility (amongst other
factors) 52,53. Moreover diets low in indigestible carbohydrates
and high in protein and/or fat typically themselves induce gut dysbiosis, pathogen
overgrowth, production of harmful metabolites, leaky gut and inflammation 22,54,55!
 On
the other hand, increasing the intake of indigestible carbohydrates, and augmenting
colonic carbohydrate fermentation, has the potential to benefit many diseases; perhaps
especially those featuring low levels of beneficial butyrate-producing bacteria
and elevated Enterobacteriaceae (e.g. IBD, IBS, Parkinson’s, arthritic
psoriasis and obesity). As discussed earlier, colonic carbohydrate fermentation
positively affects most aspects of gut and systemic health (e.g. inflammation,
immunity, insulin sensitivity and glucose tolerance), and can often even mitigate
the negative effects of a high fat or protein diet 21,22. Even conditions with SIBO may paradoxically benefit
from increased consumption of resistant starch and fibre (e.g. SIBO 56, colitis 57,58 and Crohn’s 59), perhaps in large part due to improved
intestinal motility 56,57,60.
On
the other hand, increasing the intake of indigestible carbohydrates, and augmenting
colonic carbohydrate fermentation, has the potential to benefit many diseases; perhaps
especially those featuring low levels of beneficial butyrate-producing bacteria
and elevated Enterobacteriaceae (e.g. IBD, IBS, Parkinson’s, arthritic
psoriasis and obesity). As discussed earlier, colonic carbohydrate fermentation
positively affects most aspects of gut and systemic health (e.g. inflammation,
immunity, insulin sensitivity and glucose tolerance), and can often even mitigate
the negative effects of a high fat or protein diet 21,22. Even conditions with SIBO may paradoxically benefit
from increased consumption of resistant starch and fibre (e.g. SIBO 56, colitis 57,58 and Crohn’s 59), perhaps in large part due to improved
intestinal motility 56,57,60.
In
summary, I think rather than demonising carbs, we should be asking why is
carbohydrate metabolism impaired in so many disorders, and how can we improve
it? Really all macronutrients are important, and their dietary balance can be
manipulated to support differences in our body, lifestyle and health needs. Finally
it might also be worth considering how our diet affects the wider ecosystem and
entire planet.
References
1. Schönfeld, P. & Reiser, G. Why does
brain metabolism not favor burning of fatty acids to provide energy?
Reflections on disadvantages of the use of free fatty acids as fuel for brain. J.
Cereb. Blood Flow Metab. 33, 1493–9 (2013).
2. Hardy,
K., Brand-Miller, J., Brown, K. D., Thomas, M. G. & Copeland, L. The
Importance of Dietary Carbohydrate in Human Evolution. Q. Rev. Biol. 90,
251–268 (2015).
3. Pearce,
E. L. & Pearce, E. J. Metabolic pathways in immune cell activation and
quiescence. Immunity 38, 633–43 (2013).
4. Leverve,
X., Batandier, C. & Fontaine, E. Choosing the right substrate. Novartis
Found. Symp. 280, 108–21; discussion 121–7, 160–4 (2007).
5. Hayes,
J. D. & Dinkova-Kostova, A. T. The Nrf2 regulatory network provides an interface
between redox and intermediary metabolism. Trends Biochem. Sci. 39,
199–218 (2014).
6. Stincone,
A. et al. The return of metabolism: biochemistry and physiology of the
pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. (2014).
doi:10.1111/brv.12140
7. Vasconcelos-dos-Santos,
A. et al. Biosynthetic Machinery Involved in Aberrant Glycosylation:
Promising Targets for Developing of Drugs Against Cancer. Front. Oncol. 5,
1–23 (2015).
8. Newman,
J. C. & Verdin, E. Ketone bodies as signaling metabolites. Trends
Endocrinol. Metab. 25, 42–52 (2014).
9. Kimura,
I. et al. Short-chain fatty acids and ketones directly regulate
sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc.
Natl. Acad. Sci. U. S. A. 108, 8030–5 (2011).
10. Johansson,
M. E. V, Sjövall, H. & Hansson, G. C. The gastrointestinal mucus system in
health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–61
(2013).
11. Neutra,
M. & Leblond, C. P. Synthesis of the carbohydrate of mucus in the golgi
complex as shown by electron microscope radioautography of goblet cells from
rats injected with glucose-H3. J. Cell Biol. 30, 119–36 (1966).
12. Shan,
M. et al. Mucus enhances gut homeostasis and oral tolerance by
delivering immunoregulatory signals. Science 342, 447–53 (2013).
13. Tailford,
L. E., Crost, E. H., Kavanaugh, D. & Juge, N. Mucin glycan foraging in the
human gut microbiome. Front. Genet. 6, 81 (2015).
14. Van
Tassell, M. L. & Miller, M. J. Lactobacillus adhesion to mucus. Nutrients
3, 613–36 (2011).
15. Van
den Abbeele, P. et al. Butyrate-producing Clostridium cluster XIVa
species specifically colonize mucins in an in vitro gut model. ISME J. 7,
949–61 (2013).
16. Pickard,
J. M. et al. Rapid fucosylation of intestinal epithelium sustains host–commensal
symbiosis in sickness. Nature 514, 638–641 (2014).
17. Campos,
E. et al. The yiaKLX1X2PQRS and ulaABCDEFG gene systems are required for
the aerobic utilization of L-ascorbate in Klebsiella pneumoniae strain 13882
with L-ascorbate-6-phosphate as the inducer. J. Bacteriol. 190,
6615–6624 (2008).
18. Linares,
D., Michaud, P., Delort, A. M., Traïkia, M. & Warrand, J. Catabolism of
L-ascorbate by lactobacillus rhamnosus GG. J. Agric. Food Chem. 59,
4140–4147 (2011).
19. Kasubuchi,
M., Hasegawa, S., Hiramatsu, T., Ichimura, A. & Kimura, I. Dietary Gut
Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients
7, 2839–2849 (2015).
20. Besten,
G. den et al. Short-Chain Fatty Acids protect against High-Fat
Diet-Induced Obesity via a PPARγ-dependent switch from lipogenesis to fat
oxidation. Diabetes (2015). doi:10.2337/db14-1213
21. Windey,
K., De Preter, V. & Verbeke, K. Relevance of protein fermentation to gut
health. Mol. Nutr. Food Res. 56, 184–96 (2012).
22. Simpson,
H. L. & Campbell, B. J. Review article: dietary fibre-microbiota
interactions. Aliment. Pharmacol. Ther. 42, 158–179 (2015).
23. Shen,
W., Gaskins, H. R. & McIntosh, M. K. Influence of dietary fat on intestinal
microbes, inflammation, barrier function and metabolic outcomes. J. Nutr.
Biochem. 25, 270–280 (2014).
24. Pollard,
K. What makes us human? Sci. Am. 1, (2009).
25. Perry,
G. H. et al. Diet and the evolution of human amylase gene copy number
variation. Nat. Genet. 39, 1256–60 (2007).
26. Milton,
K. The critical role played by animal source foods in human (Homo) evolution. J.
Nutr. 133, 3886S–3892S (2003).
27. Carmody,
R. N. & Wrangham, R. W. The energetic significance of cooking. J. Hum.
Evol. 57, 379–91 (2009).
28. Carmody,
R. N., Weintraub, G. S. & Wrangham, R. W. Energetic consequences of thermal
and nonthermal food processing. Proc. Natl. Acad. Sci. U. S. A. 108,
19199–203 (2011).
29. Gómez-Pinilla,
F. Brain foods: the effects of nutrients on brain function. Nat. Rev.
Neurosci. 9, 568–78 (2008).
30. Cunnane,
S. et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition
27, 3–20 (2011).
31. Pifferi,
F. et al. Long-chain n-3 PUFAs from fish oil enhance resting state brain
glucose utilization and reduce anxiety in an adult nonhuman primate, the grey
mouse lemur. J. Lipid Res. 56, 1511–8 (2015).
32. Milton,
K. Hunter-gatherer diets-a different perspective. The American journal of
clinical nutrition 71, 665–667 (2000).
33. Gurven,
M. & Kaplan, H. Longevity Among Hunter- Gatherers: A Cross-Cultural
Examination. Popul. Dev. Rev. 33, 321–365 (2007).
34. Marlowe,
F. W. et al. Honey, Hadza, hunter-gatherers, and human evolution. J.
Hum. Evol. 71, 119–28 (2014).
35. Sistiaga,
A., Mallol, C., Galván, B. & Summons, R. E. The Neanderthal meal: a new
perspective using faecal biomarkers. PLoS One 9, e101045 (2014).
36. Clemente,
F. J. et al. A Selective Sweep on a Deleterious Mutation in CPT1A in
Arctic Populations. Am. J. Hum. Genet. 95, 584–589 (2014).
37. Del
Chierico, F., Vernocchi, P., Dallapiccola, B. & Putignani, L. Mediterranean
diet and health: Food effects on gut microbiota and disease control. Int. J.
Mol. Sci. 15, 11678–11699 (2014).
38. Willcox,
D. C., Scapagnini, G. & Willcox, B. J. Healthy aging diets other than the
Mediterranean: A focus on the Okinawan diet. Mech. Ageing Dev. 136-137,
148–62 (2014).
39. Spreadbury,
I. Comparison with ancestral diets suggests dense acellular carbohydrates
promote an inflammatory microbiota, and may be the primary dietary cause of
leptin resistance and obesity. Diabetes. Metab. Syndr. Obes. 5,
175–89 (2012).
40. Armstrong,
C. W., McGregor, N. R., Lewis, D. P., Butt, H. L. & Gooley, P. R. Metabolic
profiling reveals anomalous energy metabolism and oxidative stress pathways in
chronic fatigue syndrome patients. Metabolomics (2015).
doi:10.1007/s11306-015-0816-5
41. Brown,
A. E., Jones, D. E., Walker, M. & Newton, J. L. Abnormalities of AMPK
Activation and Glucose Uptake in Cultured Skeletal Muscle Cells from
Individuals with Chronic Fatigue Syndrome. PLoS One 10, e0122982
(2015).
42. Noto,
H., Goto, A., Tsujimoto, T. & Noda, M. Low-Carbohydrate Diets and All-Cause
Mortality : A Systematic Review and Meta-Analysis of Observational Studies. PLoS
One 8, e55030 (2013).
43. Levine,
M. E. et al. Low protein intake is associated with a major reduction in
IGF-1, cancer, and overall mortality in the 65 and younger but not older
population. Cell Metab. 19, 407–17 (2014).
44. Solon-Biet,
S. M. et al. The ratio of macronutrients, not caloric intake, dictates
cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell
Metab. 19, 418–30 (2014).
45. Barbagallo,
M., Dominguez, L. J., Tagliamonte, M. R., Resnick, L. M. & Paolisso, G.
Effects of glutathione on red blood cell intracellular magnesium: relation to
glucose metabolism. Hypertension 34, 76–82 (1999).
46. Swithers,
S. E. Artificial sweeteners are not the answer to childhood obesity. Appetite
(2015). doi:10.1016/j.appet.2015.03.027
47. Chassaing,
B. et al. Dietary emulsifiers impact the mouse gut microbiota promoting
colitis and metabolic syndrome. Nature 519, 92–6 (2015).
48. Moreira,
A. P. B., Texeira, T. F. S., Ferreira, A. B., Peluzio, M. D. C. G. &
Alfenas, R. D. C. G. Influence of a high-fat diet on gut microbiota, intestinal
permeability and metabolic endotoxaemia. Br. J. Nutr. 108, 801–9
(2012).
49. Fei,
N. & Zhao, L. An opportunistic pathogen isolated from the gut of an obese
human causes obesity in germfree mice. ISME J. 7, 880–4 (2013).
50. Pimentel,
M. et al. A 14-day elemental diet is highly effective in normalizing the
lactulose breath test. Dig. Dis. Sci. 49, 73–7 (2004).
51. Staib,
L. & Fuchs, T. M. From food to cell: Nutrient exploitation strategies of
enteropathogens. Microbiol. (United Kingdom) 160, 1020–1039
(2014).
52. Bures,
J. et al. Small intestinal bacterial overgrowth syndrome. World J.
Gastroenterol. 16, 2978–90 (2010).
53. Gersemann,
M., Wehkamp, J. & Stange, E. F. Innate immune dysfunction in inflammatory
bowel disease. J. Intern. Med. 271, 421–8 (2012).
54. Russell,
W. R. et al. High-protein, reduced-carbohydrate weight-loss diets
promote metabolite profiles likely to be detrimental to colonic health. Am.
J. Clin. Nutr. 93, 1062–72 (2011).
55. Duncan,
S. H. et al. Reduced dietary intake of carbohydrates by obese subjects
results in decreased concentrations of butyrate and butyrate-producing bacteria
in feces. Appl. Environ. Microbiol. 73, 1073–8 (2007).
56. Furnari,
M. et al. Clinical trial: the combination of rifaximin with partially
hydrolysed guar gum is more effective than rifaximin alone in eradicating small
intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 32,
1000–6 (2010).
57. James,
S. L. et al. Abnormal fibre usage in UC in remission. Gut 64,
562–570 (2014).
58. Canani,
R. B. et al. Potential beneficial effects of butyrate in intestinal and
extraintestinal diseases. World J. Gastroenterol. 17, 1519–28
(2011).
59. M,
C., T, T., K, N. & M, K. High Amount of Dietary Fiber Not Harmful But
Favorable for Crohn Disease. Perm. J. 19, 58–61 (2015).
60. Rana,
S. V. et al. Relationship of cytokines, oxidative stress and GI motility
with bacterial overgrowth in ulcerative colitis patients. J. Crohns. Colitis
(2014). doi:10.1016/j.crohns.2014.01.007

Thanks this is useful stuff. I came across it doing research for a follow up post to this post https://tipsforme.wordpress.com/2016/02/16/resource-home-hacking-blood-glucose/ which I think you might like.
ReplyDelete